Enaps 미래를 위한! 세계를 위한 도약!
Business Field
BIO & Pharmaceutical Plant
Enaps Co., Ltd. provides total solution in Bio-Pharmaceutical Plant Project in compliance with cGMP. we provide the best service that comply with ASME BPE and related codes based on experiences in handling large -scale cGMP Project.


APPLICATION STANDARD
- ASME Bioprocessing Equipment
- AWS D18.2, Guide to weld discoloration levels on the inside of austenitic stainless steel tube
- ASME Boiler and Pressure Vessel Code, Section IX, Welding and Brazing Qualifications
- ASME Boiler and Pressure Vessel Code, Section V, Nondestructive Examination
- ASME B31.3, Process Piping
- ASTM A270, Specification Seamless and Welded Austenitic Stainless Sanitary Tubing
- ASTM A380, Practice, for Cleaning, Descaling, and Passivation of Stainless Steel Parts, Equipment and Systems
- FDA, 21 CFR, Parts 210 and 211, current Good Manufacturing
- cGMP current Good Manufacturing Practices
- Industrial Standards(KS, DIN, etc.)
-
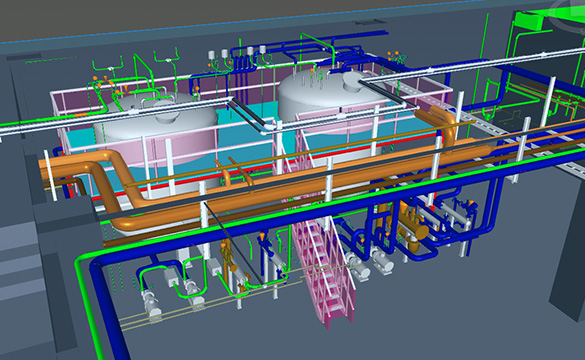
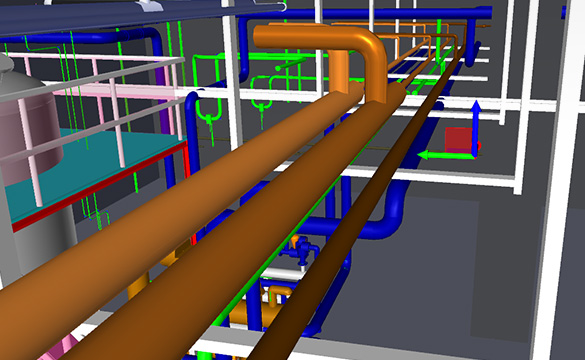
ENGINEERING
Conceptual Design / Basic Design / Detail Design / Field Engineering / Passivation Plan
-
PROCUREMENT
Equipment / Technical Evaluation / Expediting Service

-
CONSTRUCTION
Process System / Utility System / Automation


-
VALIDATION
DQ·IQ·OQ Support / E 2500 Verification Support / Documentation & Report

